

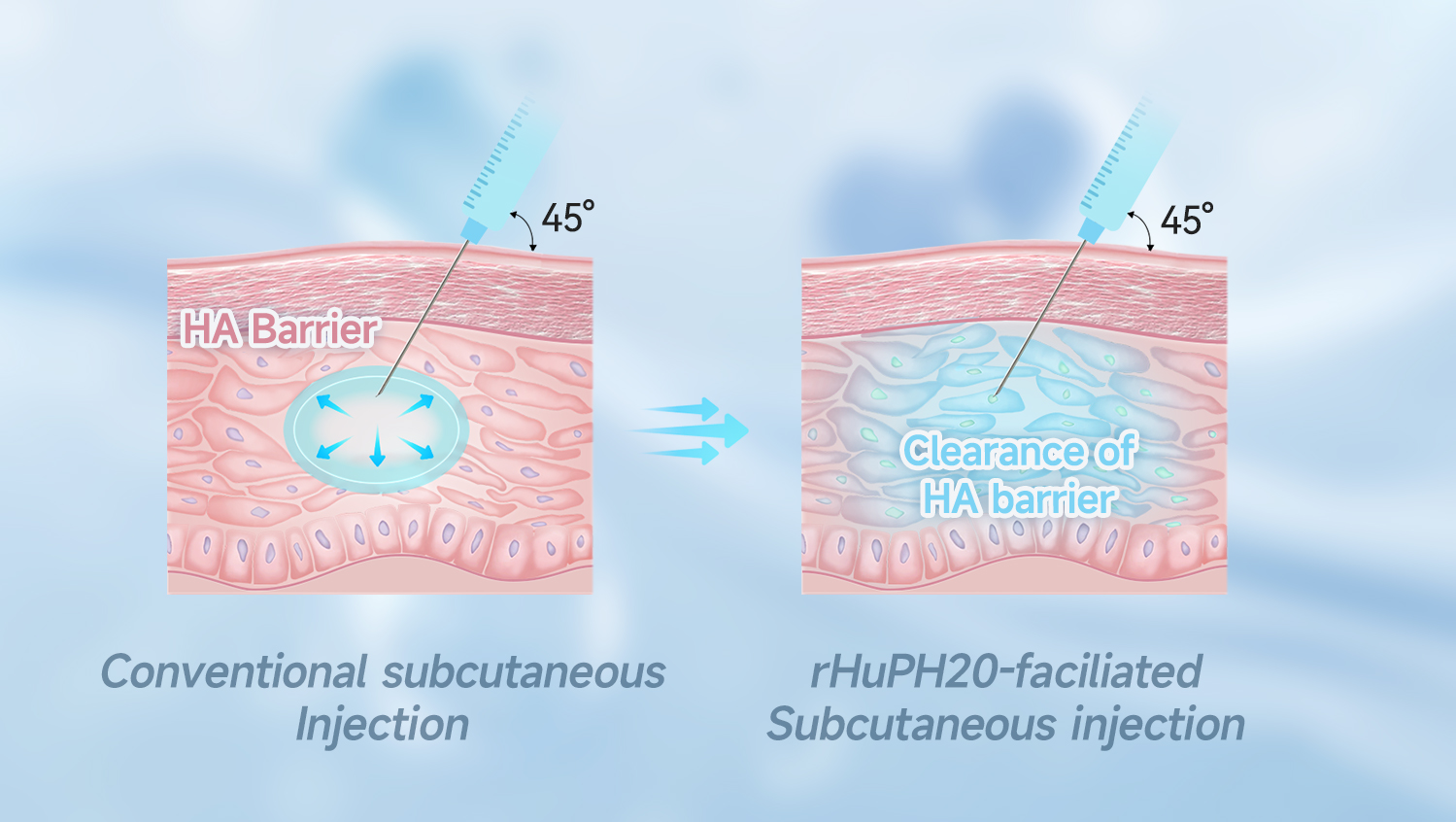
Our HYSORPTASE® is a recombinant human hyaluronidase (rHuPH20) synthesized in vitro using mammalian cells. It can locally degrade subcutaneous hyaluronic acid (HA), temporarily removing barriers to fluid flow, breaking through the subcutaneous injection volume limit of 2mL, and enabling the safe and efficient absorption of up to 1L of medication through the subcutaneous route. This improves patient experience, increases healthcare system efficiency, and enhances the competitiveness of partnered drugs.
By using HYSORPTASE® to develop co-formulations with biologics or small molecule drugs, co-formulated medications can overcome subcutaneous injection volume limitations, enabling rapid, convenient, and safe high-dose administration. This provides benefits to patients, healthcare systems, and manufacturers.
Several subcutaneous injection products using similar hyaluronidase co-formulation technology have been recognized in the global market.
HYSORPTASE® recombinant human hyaluronidase has accumulated clinical data from hundreds of patients.
In Q3 2024, the marketing application for recombinant human hyaluronidase was submitted and accepted by the NMPA.
HYSORPTASE® co-formulation: multiple self-developed and collaborative antibodies are in various stages of clinical research.
In December 2024, KJ015 (self-developed product) received clinical trial approval from the NMPA.
In June 2025, KJ015 (self-developed product) initiated Phase I clinical trials in China.
With the support of HYSORPTASE®, several products have been modified for subcutaneous infusion and are in different stages of clinical research.
In February 2025, BJ007 received clinical trial approval from the NMPA.
In August 2025, BJ007 initiated clinical trials in China.
In September 2025, BJ009 received clinical trial approval from the NMPA.
HYSORPTASE® recombinant human hyaluronidase pharmaceutical excipient registration.
In November 2024, HYSORPTASE® completed pharmaceutical excipient registration with the NMPA.
In May 2025, HYSORPTASE® completed DMF filing with the FDA.

Bao Pharma develops and produces recombinant human hyaluronidase Hysorptase® in accordance with quality management requirements for recombinant drugs. The production process complies with current GMP standards, and the production scale meets commercial demand.
Regulatory Registration Information:
China Excipient Registration Number: F20240000658
FDA DMF Number: 041587

With Hysorptase® recombinant human hyaluronidase, it becomes possible to overcome the traditional volume limitations of subcutaneous injections, offering convenient subcutaneous therapies to patients in need, either in hospitals or at home.
Hysorptase® recombinant human hyaluronidase has undergone clinical validation and has been shown to effectively enhance the dispersion of drugs subcutaneously. Additionally, several co-formulation partnership projects are in various stages of development.
 Learn more
Learn more
